IMPN: Fukuoka Classification (Guidelines)
Siegbert Faiss, Berlin
Guidelines for diagnosing and treating intraductal papillary mucinous neoplasia (IPMN) in the pancreas
Increasing numbers of cystic tumors in the pancreas are being diagnosed. It is often difficult to precisely assign these highly varied tumors to a specific entity and to distinguish them from nonneoplastic cystic lesions in the pancreas (e.g., pseudocysts). One type of lesion that is receiving particular attention — not only due to its frequency — is intraductal papillary mucinous neoplasia (IPMN) in the pancreas. These tumors have some potential for malignancy and can therefore be classified as precancerous lesions in the pancreas. This has led to prolonged debate on whether it is necessary to treat them, with conclusions ranging from surveillance to limited surgical resection up to complete pancreatectomy. Various guidelines published since 2006 have attempted to provide clear diagnostic and therapeutic recommendations for IPMNs (Sendai criteria, 2006 [1]; Fukuoka Guidelines, 2012 [2]). These were initially almost exclusively based on the size of the cystic lesion and its involvement in the main pancreatic duct and/or pancreatic branch ducts. The revised Fukuoka Guidelines [3] have now been the current diagnostic and treatment standard for these tumors since 2017.
A distinction is made in the revised Fukuoka Guidelines between Fukuoka-positive IPMNs with high-risk stigmata, IPMNs with what are known as “worrisome features,” and Fukuoka-negative IPMNs.
Fukuoka-positive IPMNs
Fukuoka-positive IPMNs are those that have high-risk stigmata for malignancy. These include IPMNs:
- In the pancreatic head that have led to obstructive icterus
- With mural nodules ? 5 mm in size that take up contrast medium
- With dilation of the main pancreatic duct to ? 10 mm (known as main duct IPMNs)
IPMNs with “worrisome features”
IPMNs with Fukuoka “worrisome features” are those with:
- Cystic lesions ? 3 cm in size
- Cystic walls that are swollen or take up contrast medium
- Dilation of the main pancreatic duct to 5–9 mm
- Abrupt changes in the diameter of the pancreatic duct and distal atrophy
- Evidence of lymph nodes
- Increased serum CA 19-9 values
- Cystic growth of at least 5 mm over 2 years
- Clinical signs of pancreatitis
Fukuoka-negative IPMNs
Fukuoka-negative IPMNs are those without high-risk stigmata and without the “worrisome features” described above.
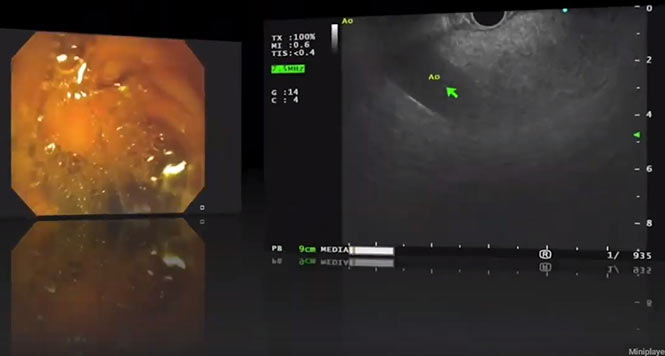
It is clear from these guideline criteria that the classification of IPMNs is now no longer carried out on the basis of their size and/or main duct or branch duct involvement but must be done in a highly differentiated way. Criteria such as contrast-absorbing mural nodules < 5 mm or ? 5 mm in size can really only be reliably assessed with high-resolution contrast-enhanced harmonic endoscopic ultrasound (CH-EUS), or less precisely with magnetic resonance imaging (MRI) (Figs. 1–5).
The guidelines also include recommendations regarding the therapeutic approach. In patients who are operable, Fukuoka-positive IPMNs should be treated surgically. In the presence of “worrisome features” and EUS evidence of mural nodules ? 5 mm, evidence of changes in the main duct, or the presence of malignancy-suspicious or even malignant cytology, surgical resection should also be aimed for. If none of that is the case, then depending on the size of the IPMN, check-ups using CT/MRI or EUS should be carried out at specific intervals. Figure 6 provides an overview of the diagnostic and therapeutic approach in IPMNs on the basis of the revised Fukuoka Guidelines [3].
An approach based on these guidelines has shown that the risk of overtreating “harmless” IPMNs due to unjustified surgery, and at the same time the development of malignant tumors out of apparently “harmless” IPMNs, is extremely low. A study including more than 1,100 patients with IPMNs showed that the rate of malignancy developing within 5 years in Fukuoka-negative IPMNs was less than 2%. In the presence of “worrisome features,” the carcinoma risk in unoperated IPMNs is approximately 4%, whereas in Fukuoka-positive patients, pancreatic carcinoma was demonstrated within 5 years in 49% [4].
When patients with an IPMN are being monitored, attention must always be given to the fact that pancreatic malignancies may also occur more frequently outside of and independently of the cystic lesions [5]. Surveillance should therefore always include the entire pancreas.






References
- Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. ‘International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas’. Pancreatology 2006; 6: 17–32.
- Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. ‘International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas’, Pancreatology 2012; 12: 183–97.
- Tanaka M, Fernandez-del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. ‘Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas’, Pancreatology 2017; 17 :738–53.
- Mukewar S, de Pretis N, Aryal-Khanal A, Ahmed A, Sah R, Enders F, Larson JJ, et al. ‘Fukuoka criteria accurately predict risk for adverse outcomes during follow-up of pancreatic cysts presumed to be intraductal papillary mucinous neoplasms’, Gut 2017 Oct; 66(10): 1811–17.
- Yamaguchi K, Kanemitsu S, Hatori T, Maguchi H, Shimizu Y, Tada M, et al.
‘Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN’, Pancreas 2011; 40: 571–80.